

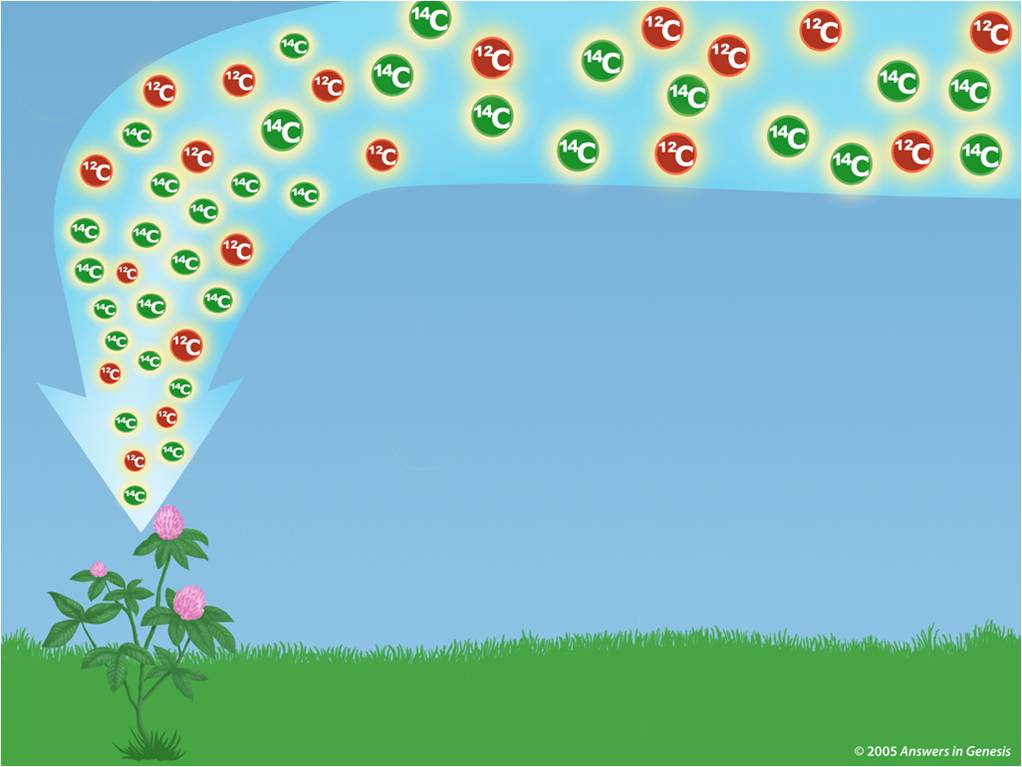
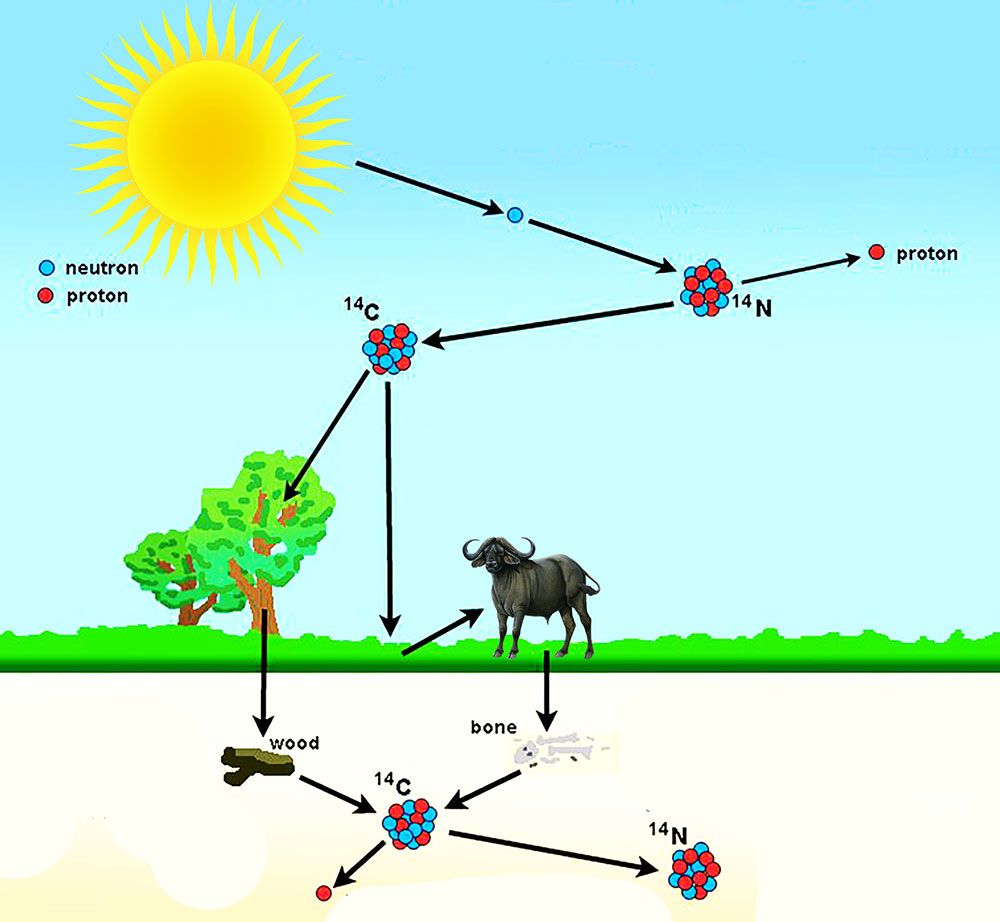
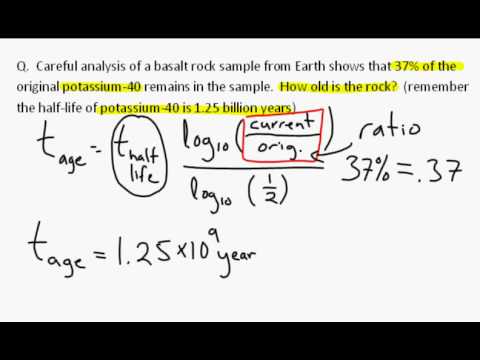
Eventually, all the carbon-14 in the remains will disappear. From that time forward, the only process at work in the body is radioactive decay. When a plant stops assimilating carbon dioxide or when an animal or human being stops eating, the ingestion of carbon-14 also stops and the equilibrium is disrupted. Although carbon-14 is radioactively decaying away in the body, it is constantly being replaced by new photosynthesis or the ingestion of food, leaving the amount relatively constant. Their bodies are said to be in “equilibrium” with carbon-14 in the air. Therefore all living plants, animals, and human beings have the same amount of carbon-14 in their bodies at the same time. Animals eat plants and/or other animals humans eat plants and animals. Living plants are active components of the overall food chain. This process is constantly ongoing, so that at any point in time the amount of carbon-14 in living plants is the same as the amount of carbon-14 in the air around them. This carbon dioxide rapidly mixes throughout the atmosphere, where at ground level it is taken in by plants during photosynthesis. Radiocarbon immediately reacts with oxygen in the air to form carbon dioxide (CO2). This leaves the amount in the air relatively constant. This carbon-14 immediately starts to radioactively decay but is constantly being recreated. A reaction occurs and a tiny number of these collisions convert nitrogen to carbon-14. Radiocarbon dating uses carbon-14 to determine the last time something (or someone) was alive.Ĭarbon-14 originates in the upper atmosphere of the earth and is created when neutrons originating from solar radiation bombardment collide with nitrogen in the air. Since it is radioactive, it gradually fades away by radioactive decay until it is all gone. Carbon-14 is present in all living things in minute amounts. Radiocarbon, or carbon-14 (also written as 14C), is an isotope of carbon that is unstable and weakly radioactive. What is Radiocarbon?Ĭarbon is the basis of life and is present in all living things. There are exceptions to the theories and relationships introduced below that are beyond the scope of this discussion. This discussion is a simplified introduction to radiocarbon dating. One industrial application of radiocarbon dating is ASTM D6866.Anything that is more than 50,000 years old no longer has carbon-14.Radiocarbon, or carbon-14, is present in all living and recently expired matter.Call (305) 662-7760 or fill out our sample form today if you’re ready to send samples for testing. BETA has been the world leader in Carbon-14 analyses since 1979 and has unmatched expertise analyzing complex samples. After hours upon hours of digging, you strike gold (not literally).Headquartered in Miami, Florida, Beta Analytic provides biobased content / renewable carbon measurements to top commercial organizations, government agencies, scientists and engineers. Imagine you are an archeologist at a dig site in Rome. Saturated Unsaturated and Supersaturated.Reaction Quotient and Le Chatelier's Principle.Prediction of Element Properties Based on Periodic Trends.Molecular Structures of Acids and Bases.
Variable Oxidation State of Transition Elements.Transition Metal Ions in Aqueous Solution.Single and Double Replacement Reactions.


 0 kommentar(er)
0 kommentar(er)
